A previous article showed that the electrostatic term of the lattice energy of a crystal contains a factor (A) that depends on the type of crystalline reticulum. This article will look more in detail how to calculate this term and its value for a simple ionic system.
The total Coulomb interaction energy of a crystal is given by the sum of the single pair interaction terms:
for ions with charges qA and qB and distance rAB. The sum extends to all pairs of ions present in the solid for any crystalline structure.
The sum converges very slowly because the first neighbours give a first substantial contribution with a negative summation term. Second neighbour atoms produce a slightly weaker positive term. It then continues to infinity with terms of alternating signs by smaller and smaller values. In this way, the resulting effect will be that the attraction between cations and anions predominates and provides a favourable negative contribution to the solid’s energy.
Infinite one-dimensional reticulum.

In a mono-dimensional reticulum with alternate cations and anions at intervals of constant of length d, and having charges qA=+Z and qB=-Z, the interaction of one ion with all others is given by the series:
(the factor 2 derives from the fact that each ion in the reticulum had on both sides identical ions). The sum depends only on the type of reticulum and by the distance d between the centres of adjacent particles:
In the case of one-dimensional lattice, contain the term
depending on the charge type and reticulum. The factor 2ln 2 (= 1,3862944) is called Madelung (A) constant and characterizes the reticulum’s symmetry.
Infinite two-dimensional reticulum
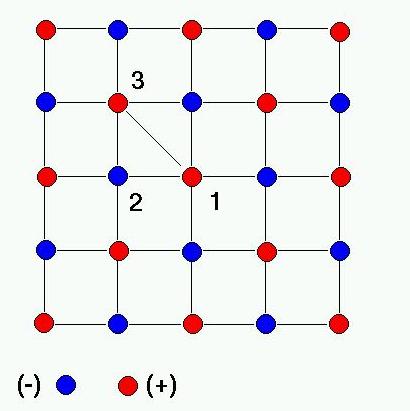
In the case of a two-dimensional reticulum, the cations and the anions are disposed at regular intervals on contiguous squares of length d and with e
. Considering as centre the ion (1) in the above diagram and moving radially from it, the interaction of this ion with all the others is given by:
Infinite three-dimensional reticulum
This formulation can be easily extended to a three-dimensional lattice.

In a simple solid, like the NaCl in the figure, the Madelung constant depends on the crystal type and by inter-ionic distances. The Madelung constant for a three-dimensional lattice is calculated by starting from an ion placed at the lattice’s centre and then moving radially until the first neighbours, having charges of opposite value, at distance
are found. Then, by moving radially to the central ion, the second
neighbours (having the same electric charges of the central one) are located at the distance
. The procedure is then further extended in the same way for a group of neighbour atoms organized in a concentric shell around the atoms’ previous group. The Madelung constant, in this case, will be defined by the summation:
,
where indicates the sign of each term of the sum, and it is positive if the ions have opposite electric charges (attraction) and negative in sign if they have an equal charge (repulsion), and
+
is the sum of ionic radii of the ions in the lattice.
Example
As an example, we consider the Na+ in NaCl. It has 6 first neighbour’s Cl– (n1 = 6 at a distance) d1=d; then there are 12 second Na+ as second neighbours (n2=12) at a distance ; 8 third neighbours Cl– (n3=8) at a distance ; etc. Therefore, the resulting series is given by the following expression:
The potential energy for one mole in the crystalline structure is given by:
| Structure | A | A/n* | Coordination |
| CsCl | 1.763 | 0.88 | (8,8) |
| Halite (NaCl) | 1.748 | 0.87 | (6,6) |
| Fluorite (CaF2) | 2.519 | 0.84 | (8,4) |
| Wurtzite (ZnS) | 1.641 | 0.82 | (4,4) |
| CdCl2 | 2.244 | 0.75 | (6,3) |
| CdI2 | 2.191 | 0.73 | (6,3) |
| Rutile ( | 2.408 | 0.80 | (6,3) |
| Curundum ( | 4.172 | 0.83 | (6,4) |
* This this the value of A divided by the number n of ions in the chemical formula of the salt.
As shown for the NaCl (6,6) and CsCl (8,8) structures, the constant of Madelung increases with the coordination number as the most important contribution comes from the first neighbours.
If you want to read more about crystal structures and their energetics then you might be interested to these two articles:
If you have found interesting and useful my article, do not forget to press “Like it” and subscribe for updates on new ones!

Very nice presentation to introduce someone to the topic. Well written, concise, and emphasizing both the fundamentals and numerical values. Compared to the “hair grind” summations on Wolfram or the precise but unclear discussion on Wikipedia this posting is where I would recommend a student start reading.
LikeLiked by 2 people
Pingback: CRYSTAL STRUCTURES |
Pingback: Madelung常数的计算 |
Pingback: Madelung定数の計算 |
Pingback: El cálculo de la constante de Madelung |