Proteins are not always rigid structures. Many of their most important parts — linkers, loops, and disordered regions — are highly flexible, constantly changing shape in solution. To understand how these regions behave, scientists often study short model peptides that capture the essential physics of flexibility. In a new article [1], I have explored the behavior of glycine- and serine-rich octapeptides using molecular dynamics simulations combined with concepts from FRET (Förster Resonance Energy Transfer) spectroscopy (see also my previous post).
A Tiny Fluorophore with Big Potential
The study focuses on peptides labeled with 2,3-diazabicyclo[2.2.2]oct-2-ene (DBO), a very small and sensitive fluorescent probe. Paired with tryptophan, DBO allows researchers to measure extremely short distances, making it ideal for probing compact and flexible peptides that are otherwise difficult to study experimentally.
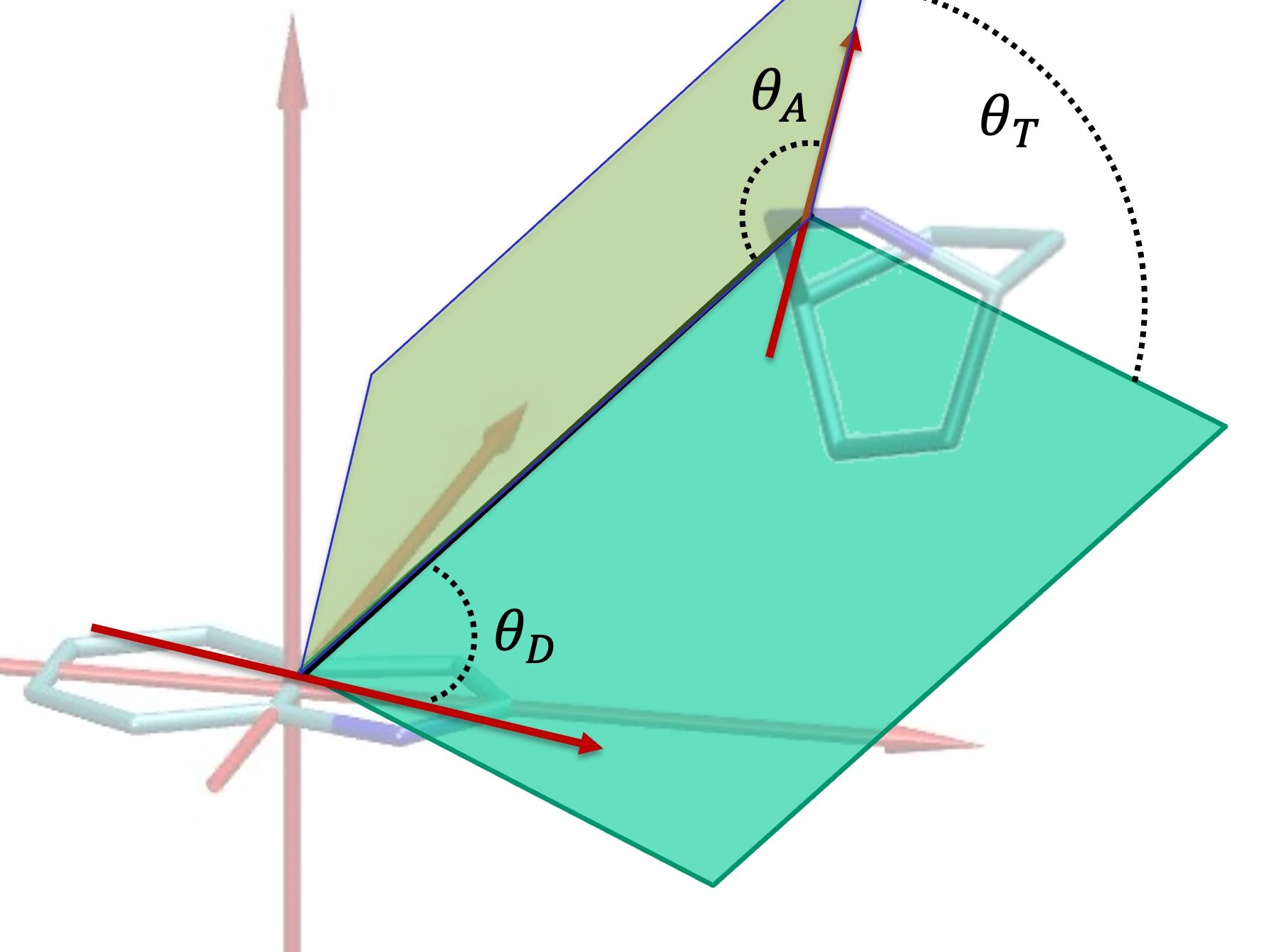
What Do These Peptides Really Look Like?
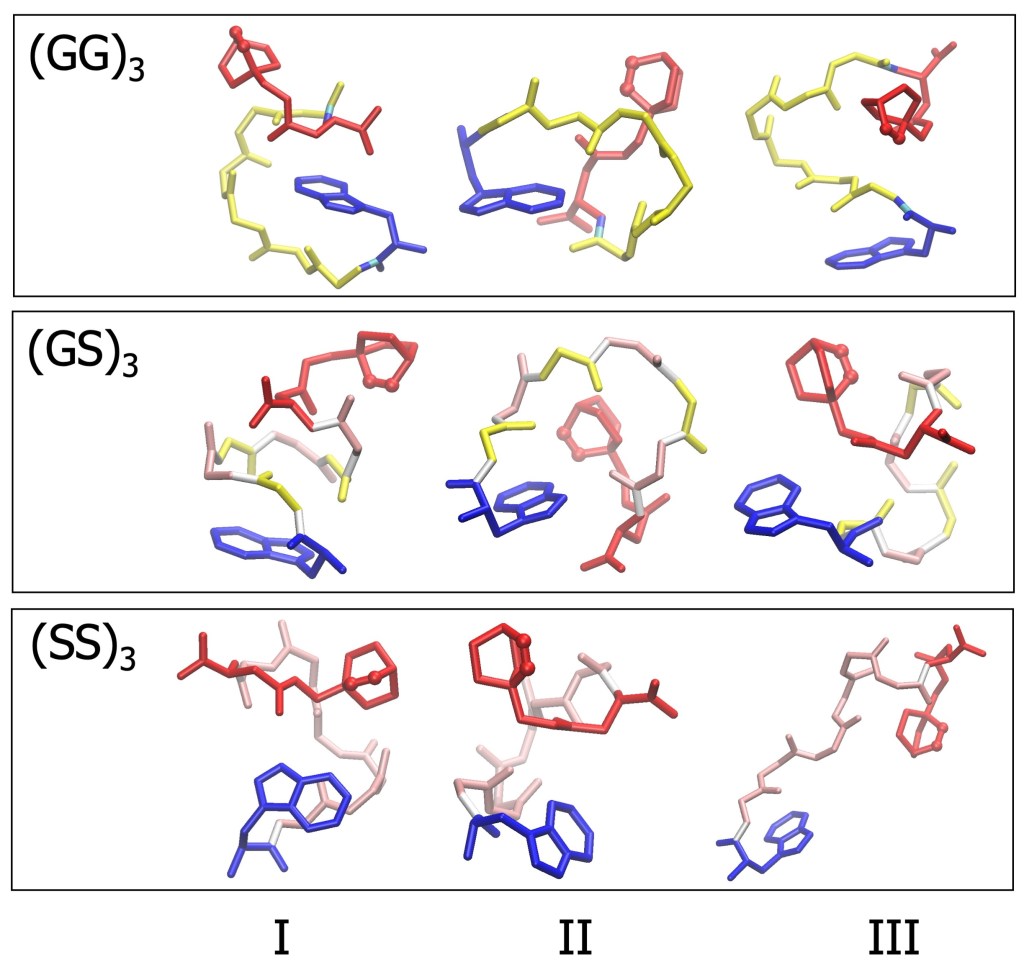
Although glycine- and serine-rich peptides are often described as random coils, our simulations reveal a more nuanced picture:
- The peptides are largely disordered, with no stable secondary structure
- At the same time, they tend to adopt surprisingly compact shapes in water
- Only a few dominant conformations account for most of the observed behavior
Small differences in amino-acid composition matter only slightly, with serine-rich chains showing a modest tendency to stretch more than glycine-rich ones.
Why It Matters
By comparing simulated distances and orientations with experimental FRET data, the study confirms that modern molecular simulations can reliably reproduce real spectroscopic measurements. This strengthens the connection between experiments and molecular models, helping us better understand flexible protein regions that play key roles in biology.
REFERENCE
[1] D. Roccatano. A Molecular Dynamics Simulation Study of Glycine/Serine Octa Peptides Labeled with 2,3-diazabicyclo [2.2.2] oct-2-ene Fluorophore. J. Chem. Phys.,160 (14), (2024). DOI: https://doi.org/10.1063/5.0190073
