Boron is the fifth element in the periodic table. It is also the first element of the boron group or the group thirteenth of the periodic table. It is a metalloid element, meaning its properties are between a metal and a nonmetal. The chemical symbol for boron is B, which has an atomic weight of 10.81 grams per mole.

In its ground state, a boron atom contains five electrons arranged in two energy levels. The first energy level, or shell, can hold up to two electrons, while the second can hold up to eight. However, boron has three valence electrons, meaning only the first two energy levels are filled, leaving the third energy level partially empty.
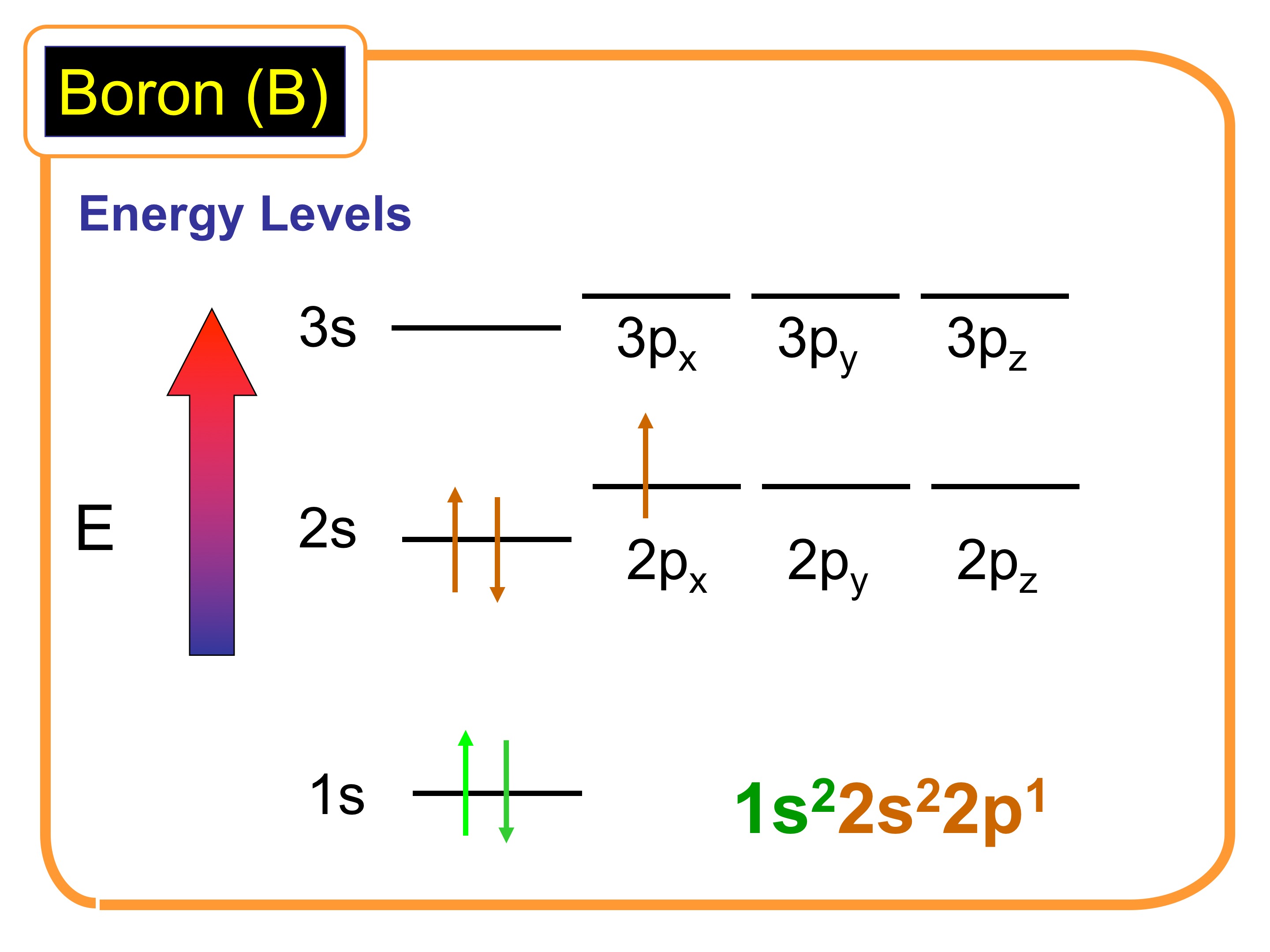
The electronic structure of boron can be represented using an Aufbau diagram. In the diagram, two electrons are in the first shell (1s) while the three valence electrons are in the second shell.
Boron is a unique element with various applications in different fields. It is widely used in the production of ceramics, glass, and polymers. It is also an essential component in the semiconductor industry, where it is used in the manufacturing of electronic devices. Additionally, boron compounds have applications in the field of medicine, agriculture, and nuclear energy.
Due to its electronic properties,, the boron atom’s electronic structure and properties make it a fascinating element with diverse uses in various scientific and industrial fields.
Boron atoms can combine, carbon and hydrogen atoms forming ionic hydride clusters of different dimensions with distinctive structures. For example, in the picture below, it is shown one of such compounds composed of 12 atoms of boron atoms bonded to 12 hydrogen atoms with the formula . This stable compound is called dodecaborane anion, and it has the geometry of the regular icosahedron.

The regular icosahedron is a convex polyhedron with 20 faces, 30 edges, and 12 vertices. It is one of the five Platonic solids with the most sides.
The dodecaborate cluster B12H122− and its derivatives are weakly coordinating ions that have found numerous applications in various fields. One prominent area of application is boron neutron capture therapy, where these compounds play a role in targeting cancer cells for treatment. Additionally, they are used in the development of ionic liquids, which are gaining attention due to their unique properties and potential applications in various industries. Furthermore, these derivatives have shown promise in molecular imaging techniques, particularly in the context of nonlinear optical spectroscopy.
Interestingly, derivatives of the dodecaborate cluster have also demonstrated the ability to bind to lipid membranes and influence their structure and tightness. This property has implications for understanding membrane dynamics and the modulation of biological processes. Moreover, these boron cluster compounds have been recognized as enzyme inhibitors, highlighting their potential in therapeutic interventions.
Despite their wide range of applications, the interaction of boron clusters with biological interfaces, such as lipid bilayers or protein surfaces, remains not fully understood. This knowledge gap hinders the progress in fully harnessing the potential of these compounds. Another aspect that has yet to be thoroughly explored is the detailed structure of the hydration shell of these anions in an aqueous environment. While a few experimental studies have identified the presence of a peculiar dihydrogen bond between the boron hydrogen atoms of the anions and proton donors like methanol, a comprehensive understanding of hydration dynamics is still lacking.
Considering the importance of these compounds, we have developed new molecular models for molecular dynamics (MD) simulations of various boron cluster anions (Figure below). These models are optimized by comparing them against experimental water/1-octanol partition coefficient data, allowing for investigations into the interaction of the molecules with biological interfaces such as lipid layers.

The force field has been published in the Inorganic Chemistry Journal [1]. Here are shown some examples of the results [1].

(a) amino triethyl- derivative, (b) amino trimethyl, (c) and (d) unfunctionalized boron anion. Yellow and blue isosurfaces represent the distribution of the hydrogen and oxygens atoms, Respectively. Isosurface values have been chosen to show the first solvation shell distinctly. Right panel: Final equilibrated configuration from the QM calculations on an MD-generated water cluster around the B12H12 anion. The indicated H-H bond distances are in Å.
REFERENCES
- K. Karki, D. Gabel, D. Roccatano. A water solvation model of dodecaborate clusters. Inorg. Chem. 51 (9), 4894–4896 (2012).
