The electronegativity of a chemical element measures the tendency of an atom to attract electrons around it. This definition was formalized for the first time, in a semi-empirical form, by the chemist Linus Pauling in the early 1930s, but it had already been proposed in the late 1800s by the Swedish chemist Berzelius. In molecules, this tendency determines the molecular electronic distribution and therefore influences molecular properties such as the distribution of partial charges and chemical reactivity. Pauling provided an electronegativity scale by comparing bond dissociation energies of pairs of atoms (A, B) using the equation
With $E_{AB}$, $E_{AA}$, and $E_{BB}$ being the dissociation energies of the molecules AB, AA, and BB, respectively.
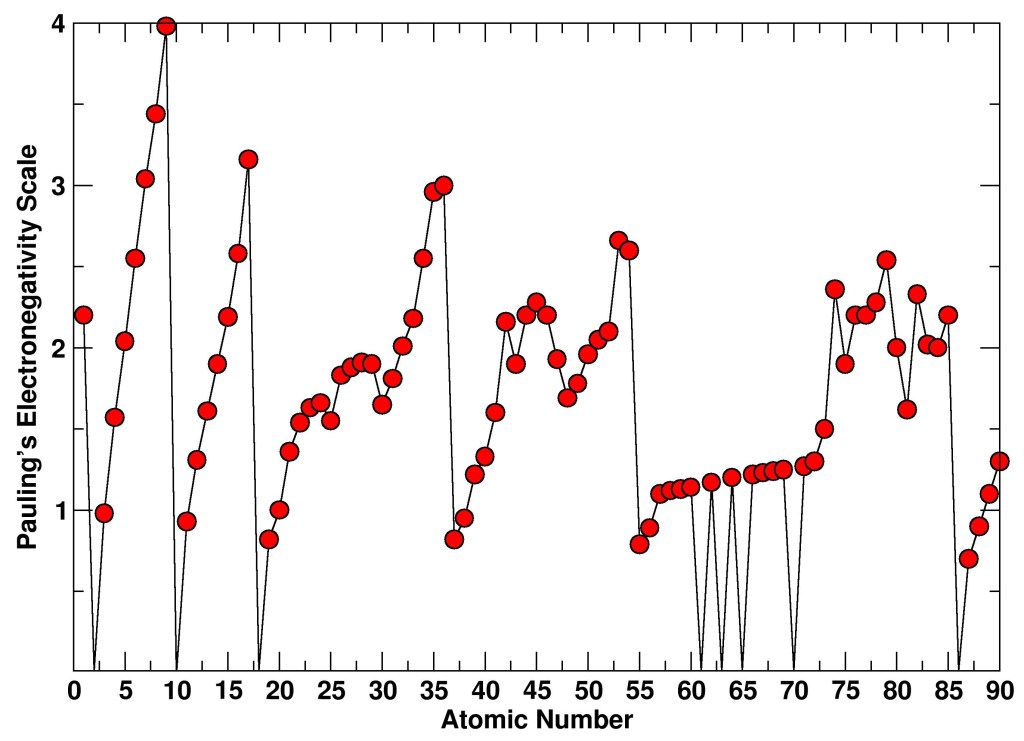
A few years later, in 1934, Mulliken proposed an expanded definition of electronegativity based on spectroscopically measurable atomic properties such as ionization potential (I) and electron affinity (E):
